Good Morning: Clinical Trial
Background
As the increase in alcohol consumption grows, the demand for effective hangover remedies grows. However, many over-the-counter hangover treatments lack scientific validation, leaving consumers searching for reliable solutions to alleviate their hangover symptoms. Despite the abundance of commercially available products, most have not undergone rigorous clinical testing to confirm their effectiveness in relieving hangover symptoms.
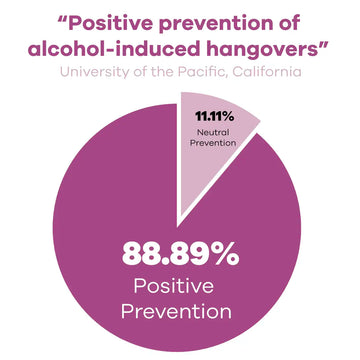
The Results
The Hangover Secret (our old company name) showed “positive signals in the prevention of alcohol-induced hangover, especially headaches”
The improvements that participants say, surpassed the minimum clinically significant difference in overall AHS score and three individual AHS symptoms scores (hangover, headache, and thirst).
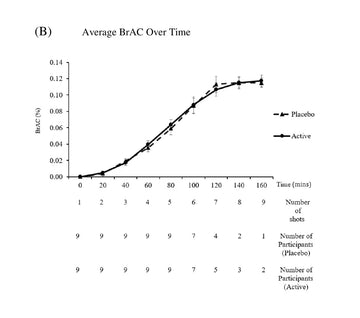
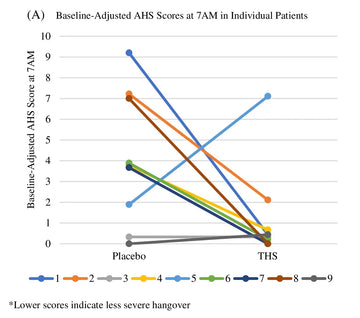
Nine participants completed the study. AHS scores increased from baseline to 7 AM by 4.11 ± 3.17 and 1.26 ± 2.29 for the placebo and active arms respectively (P = .16). AHS headache scores increased from baseline to 7 AM by 2.44 ± 1.67 and 1.11 ± 1.17 for the placebo and active arms respectively (P = .06). AHSS scores increased from baseline to 7 AM by 1.0 ± 1.05 and 0.41 ± 1.08, for the placebo and active arms respectively (P = .30). There was no significant difference between average BrAC at 7 AM between the placebo and active arms.







